- HOME
- Vienna 2024
- Lithuania 2023
- 16th CEEPC Prague 2022
- Proteome & Proteomics
- Proteomics Potentiality
- Precision Medicine & Cancer
- Proteomics and COVID-19
- Big Data & AI
- Spotlight Lithuania
- Humanity
- Meeting Reports & Tributes
- Country Profiles
- Proteomic Snippets
- Enabling Advances
- Spotlight Czech Republic
- Spotlight Poland
- 13th CEEPC - Ustron, Poland
- Spotlight Romania
- 12th CEEPC - Bucharest
- Spotlight Slovakia
- Spotlight Macedonia
- Sports Medicine
- Contacts & Copyrights
Proteomics & Viruses - COVID - 19 challenges
.....now Virology related proteo-genomics!
Vaccine development / Viral Proteomics
Diagnostic testing / Viral Surveillance
Cytokine Storm & Drug Treatments
Vaccine Safety Testing
Vaccine war & Vaccine Nationalism
Virus mutations
 CEEPC has been focused on 'Global interconnectivity, data & expertise sharing' for many years .............ironically, COVID - 19 is also driving this ideology !
CEEPC has been focused on 'Global interconnectivity, data & expertise sharing' for many years .............ironically, COVID - 19 is also driving this ideology !
Journals, Publications, Authors & Graphics acknowledged where materials is used or quoted

Photo - Reuter
COVID-19 Reality - Overview by Suresh Jivan Gadher
Sadly, COVID has exposed the fragility of our hospitals, disregard for our core health care workers and a lack of tools, training and often PPE. It has also challenged the way we take care of our elders, families, friends and colleagues. As regards the 'rich - poor' divide, it has exposed one's selfishness, ignorance and attitudes that lay hidden or dormant. It has made us question humanity or lack of it!
With reference to Global Health, there remains an urgent need to address the lack of 'vaccine equity' or 'vaccine equality' and a need to share vaccines in a much fairer way. Currently, there is no 'universal health coverage', meaning that millions of poor people in many parts of the world will have to go without vaccination. COVID has highlighted a key need which is to 'work in solidarity' where no one is left behind.
The pandemic's long term damages will haunt us in years to come. It is obvious that besides millions of deaths, various pressing health issues such as mental health, stress, Long COVID, damage to the organs, loss of faculties and complications to newborns, are going to be the challenges of the future.
Technology wise, COVID has introduced 'Tele-medicine', 'Zoom consultations', 'self -testing', 'digital diagnosis', 'AI driven population monitoring' and 'robotic interactions'. All these and many more technological advances have highlighted the power of various technololgies from a simple mobile phone to a complex 'digital pill' for health monitoring.
-
I don’t think we are going to eradicate COVID-19 or its' variants in a hurry. As evidenced by Science and Medicine, we have managed to eradicate only one virus, which was smallpox. Like with polio, measles and rubella, vaccines have helped to eliminate these diseases in some parts of the world. None of these diseases have been eliminated globally, and there have been measles outbreaks and rubella cases in recent years. Efforts to increase vaccination rates are critical for maintaining elimination of these diseases. In a similar fashion, we will need to do this with COVID-19 globally if we are to get any degree of success.
-
At some point in time, we may have enough control over the COVID- 19 virus. In fact, we won’t be able to get a much greater control than we have right now and this is not much of a control level to be proud of. We have managed to get to this level of control by following good hygiene, wearing masks, vaccinating and recently, by boosting our resistance to the virus. We now need to get to a very very low level and to a point where the virus does not interfere with our everyday life, work and other activities including our economy, schools and University education. As the exponential growth of the Omicron variant dampens, increased surveillance for the next variant may stand us in good stead in coming months.
-
Hopefully, with better and more effective vaccines, new antibody and anti-viral COVID drugs and improved AI monitoring of virus mutation, it may start to look like a ‘flu’ we see each year and which we take in our stride.
Suresh Jivan Gadher
 Vaccine efficacy & Viral mutations
Vaccine efficacy & Viral mutations

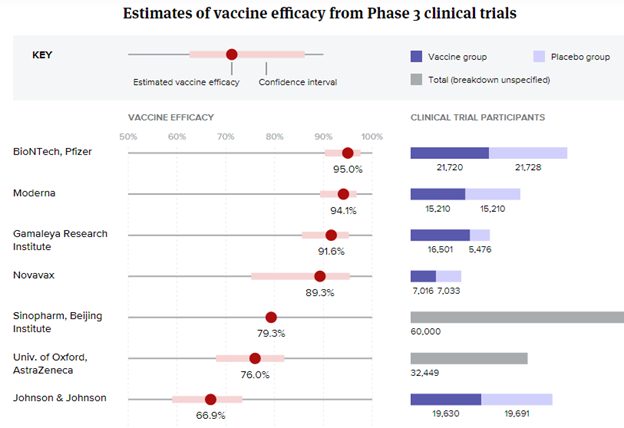
Credit: BioPharmaDive - Jonathan Gardner, Ned Pagliarulo & Ben Fidler
 Vaccines to date
Vaccines to date
Sinopharm Vaccine
The pandemic emerged in China first and the first COVID-19 vaccine was produced there. Its efficacy is high (around 72%) and one dose is enough.
Sputnik V Vaccine
The Russian vaccine was produced at full speed and provoked mistrust among some countries. However, it has been shown to be over 90% effective and one dose is all it takes.
Moderna
The Moderna vaccine was one of the first to be introduced and is 95% effective and is being given to all of the populations, especially among the elderly. It requires two doses.

Pfizer-BioNTech Vaccine
Very similar in characteristics to the Moderna vaccine with 95% efficacy, requires two doses. Storage at very low temperature (-80 degrees C) is required.

AstraZeneca Vaccine
Breaking news - US clinical trial study update at 22 March 2021
https://clinicaltrials.gov/ct2/show/NCT04516746?term=NCT04516746&draw=2&rank=1.
The AstraZeneca US Phase III trial of AZD1222 demonstrated statistically significant vaccine efficacy of 79% at preventing symptomatic COVID-19 and 100% efficacy at preventing severe disease and hospitalisation. This interim safety and efficacy analysis was based on 32,449 participants accruing 141 symptomatic cases of COVID-19. The trial had a 2:1 randomisation of vaccine to placebo. Vaccine efficacy was consistent across ethnicity and age. Notably, in participants aged 65 years and over, vaccine efficacy was 80%.
The vaccine was well tolerated, and the independent data safety monitoring board (DSMB) identified no safety concerns related to the vaccine. The DSMB conducted a specific review of thrombotic events, as well as cerebral venous sinus thrombosis (CVST) with the assistance of an independent neurologist. The DSMB found no increased risk of thrombosis or events characterised by thrombosis among the 21,583 participants receiving at least one dose of the vaccine. The specific search for CVST found no events in this trial.
AZ vaccine has the advantage that it doesn’t require transportation in deep-freeze conditions and can be stored at 4 degrees C. WHO, FDA, EMA & MHRA have, once again, given their approval and safety for use.

Johnson & Johnson Vaccine
J & J vaccine requires a single dose and has more than 70% efficacy. It is easy to transport without the need for extreme cold temperature.
CureVac / Bayer Vaccine
Many vaccines are still in the pipeline for example, Bayer Pharmaceuticals is working with CureVac. France, Spain, India and Japan too are working on novel vaccines.

Vaccination is the only route to end the pandemic and it will ensure that COVID-19 deaths can be avoided and that, at most, milder forms of the infection will occur. It seems clear that those vaccinated are protected to the extent that, even with new variants, they would not suffer from the most severe forms of coronavirus. Currently, the Moderna and Pzifer-BioNTech vaccines are effective against the South African variant but it is difficult to say if other variants (Brazilian, Californian, Kent,......) could circumvent immunization.
Scientists and Pharmaceutical companies are already working on new vaccines that would immunize against the new COVID-19 variants. Eventually, the pandemic is likely to evolve into something like the flu, where you have to be vaccinated every season because the virus would changes a little every year.
The complexity of COVID-19 will keep the scientific community experimenting. As with any medication or immunisation, there may be side-effects. Being immunized by the vaccine does not mean that one cannot transmit the virus to others. What the vaccine does is slow down the ability of the virus to make one sick, but scientists suspect that transmission is still possible. So even with mass vaccination, the safety mask is still going to be part of the regular every day life moving forward.
 SARS-CoV-2 Variant & the Latin American Variant
SARS-CoV-2 Variant & the Latin American Variant
(SARS-CoV-2 VUI 202012/01 (Variant Under Investigation, year 2020, month 12, variant 01)
The new VUI-202012/01 variant has been identified in several countries including Australia, Denmark, Italy, Iceland and the Netherlands. The variant is defined by the presence of a range of 14 mutations resulting in amino acid changes and three deletions. Some of these mutations may influence the transmissibility of the virus in humans. Preliminary reports by the UK are that this variant is more transmissible than previous circulating viruses, with an estimated increase of between 40% and 70% in transmissibility (adding 0.4 to the basic reproduction number R0, bringing it to a range of 1.5 to 1.7). Laboratory studies are ongoing to determine whether these variant viruses have different biological properties or alter vaccine efficacy. There is not enough information at present to determine if this variant is associated with any change in severity of clinical disease, antibody response or vaccine efficacy.
 COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses
COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses
Sahin, U., Muik, A., Derhovanessian, E. et al. Nature 586, 594–599 (2020).
https://doi.org/10.1038/s41586-020-2814-7
 Oxford Vaccine - a success !
Oxford Vaccine - a success !

The Oxford vaccine is made by taking a common cold virus (adenovirus) from chimpanzees and deleting about 20 per cent of the virus’s instructions. This means it is impossible for the vaccine to replicate or cause disease in humans, but it can still be produced in the laboratory under special conditions. By removing these genetic instructions there is space to add the instructions for the spike protein from SARS-CoV-2. Once inside a human cell the genetic instructions for the spike protein need to be ’photocopied’ many times – a process known as transcription. In any vaccine system, it is these ’photocopies’ that are directly used to make large amounts of the spike protein.
Once the spike protein is made, the immune system will react to it and this pre-trains the immune system to identify a real COVID-19 infection. So, when the person vaccinated is confronted with the SARS-CoV-2 virus their immune system is pre-trained and ready to attack it. Adenoviruses have been used for many years to make vaccines, and these are always tested to very high standards to make sure every batch of vaccine has the correct copy of genetic instructions embedded in the vaccine. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects, from 1 week to 90 years of age, in vaccines targeting over 10 different diseases.
Thanks to very recent advances in genetic sequencing and protein analysis technology, researchers were for the first time also able to directly check thousands and thousands of the 'photocopied' instructions produced by the Oxford vaccine within a cell. In this way they were able to directly validate that the instructions are copied correctly and accurately, providing greater assurance that the vaccine is performing exactly as programmed. At the same time, the researchers checked the spike protein being made by the vaccine inside human cells also accurately reflects the instructions as programmed.
 Vaccine Testing for safety
Vaccine Testing for safety
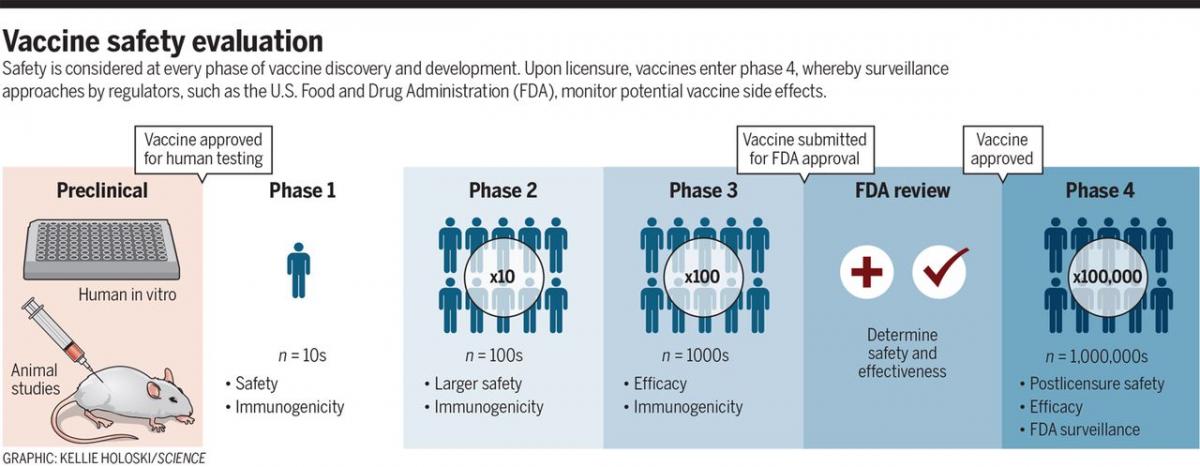
Credit: David M. Knipe, et. al, DOI: 10.1126/science.abf0357
There is an urgent need for COVID-19 vaccines and exciting progress to that end, but there remains a critical public health obligation to conduct rigorous evaluation to ensure safety as well as efficacy. Vaccines remain one of the most successful biomedical tools for prevention of disease. The urgent need for COVID-19 vaccines must be balanced with the imperative of ensuring safety and public confidence in vaccines by following the established clinical safety testing protocols throughout vaccine development, including both pre- and postdeployment.
 Vaccine Progress to date - November 2020
Vaccine Progress to date - November 2020
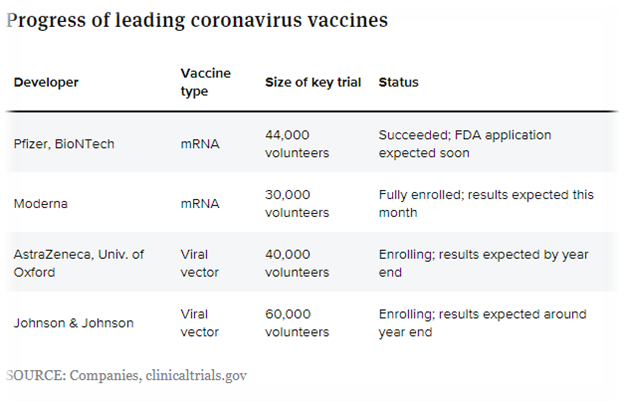
Credit - Jonathan Gardner - BioPharm Dive
 SARS-CoV-2 vaccines: a variety of approaches
SARS-CoV-2 vaccines: a variety of approaches
Nature 580, 576-577 (2020) doi: 10.1038/d41586-020-01221-y
All vaccines aim to expose the body to an antigen that won’t cause disease, but will provoke an immune response that can block or kill the virus if a person becomes infected. There are at least eight types being tried against the coronavirus, and they rely on different viruses or viral parts.
Virus vaccines
At least seven teams are developing vaccines using the virus itself, in a weakened or inactivated form. Many existing vaccines are made in this way, such as those against measles and polio, but they require extensive safety testing. Sinovac Biotech in Beijing has started to test an inactivated version of SARS-CoV-2 in humans.
Viral-vector vaccines
Around 25 groups say they are working on viral-vector vaccines. A virus such as measles or adenovirus is genetically engineered so that it can produce coronavirus proteins in the body. These viruses are weakened so they cannot cause disease. There are two types: those that can still replicate within cells and those that cannot because key genes have been disabled.
Nucleic-acid vaccines
At least 20 teams are aiming to use genetic instructions (in the form of DNA or RNA) for a coronavirus protein that prompts an immune response. The nucleic acid is inserted into human cells, which then churn out copies of the virus protein; most of these vaccines encode the virus’s spike protein.
Protein-based vaccines
Many researchers want to inject coronavirus proteins directly into the body. Fragments of proteins or protein shells that mimic the coronavirus’s outer coat can also be used.
Industry trials
More than 70% of the groups leading vaccine research efforts are from industrial or private firms. Clinical trials start with small safety studies in animals and people, followed by much larger trials to determine whether a vaccine generates an immune response. Researchers are accelerating these steps and hope to have a vaccine ready in 18 months.
Schematics and Information: Nature 580, 576-577 (2020) doi: 10.1038/d41586-020-01221-y
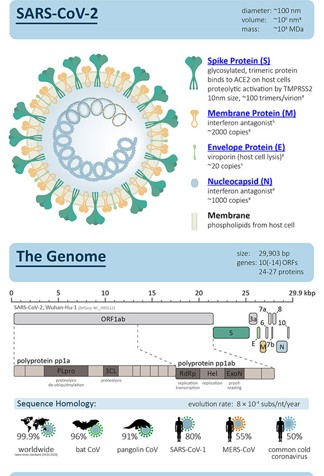
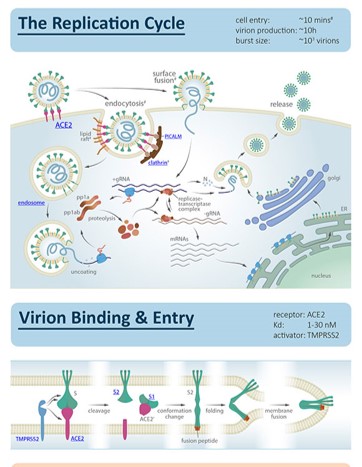
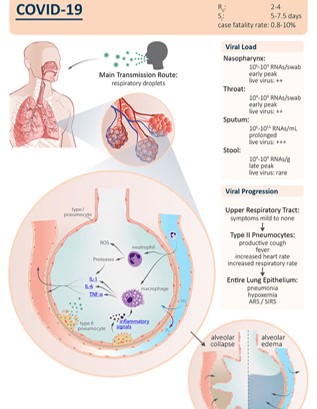
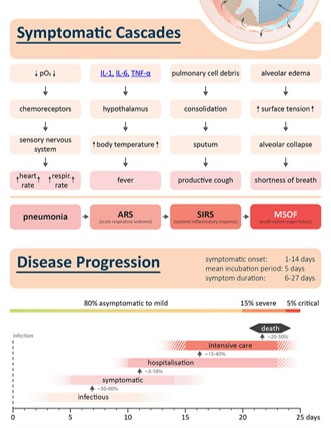
 COVID - 19 Story in a 'nutshell'
COVID - 19 Story in a 'nutshell'
 Race to find COVID-19 treatment - Solidarity approach
Race to find COVID-19 treatment - Solidarity approach
Kai Kupferschmidt1, Jon Cohen2, Science 27 Mar 2020: Vol. 367, Issue 6485, pp. 1412-1413
DOI: 10.1126/science.367.6485.1412
With cases of the new coronavirus disease 2019 (COVID-19) climbing steeply everywhere from Madrid to Manhattan , overwhelming one hospital after another and pushing the global death toll past 17,000, the sprint to find treatments has dramatically accelerated. Drugs that stop the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), could save the lives of severely ill patients, protect health care workers and others at high risk of infection, and reduce the time patients spend in hospital beds. Below is a schematic of the way the virus attacks and the lines of intervention.
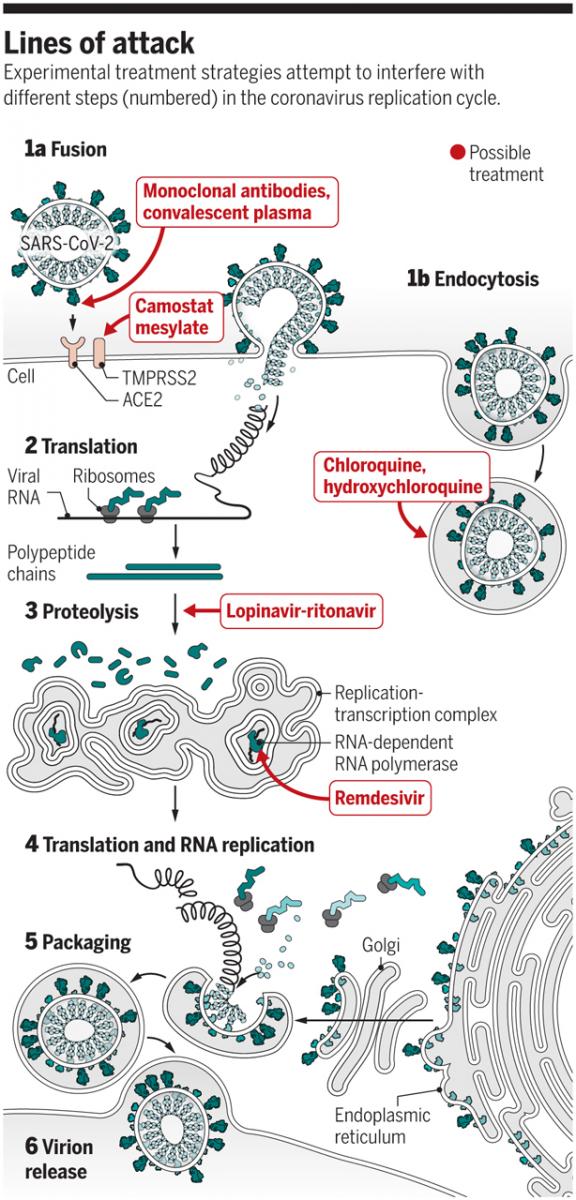
Graphic: V. Altounian / Science - Lines of attack and viral replication
 Cytokine release syndrome in severe COVID-19 - John B. Moore1 and Carl H. June2. 1Department of Hematology-Oncology, Walter Reed National Military Medical Center, Bethesda, MD, USA. 2Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, PA, USA.
Cytokine release syndrome in severe COVID-19 - John B. Moore1 and Carl H. June2. 1Department of Hematology-Oncology, Walter Reed National Military Medical Center, Bethesda, MD, USA. 2Center for Cellular Immunotherapies, University of Pennsylvania, Philadelphia, PA, USA.
J. B. Moore, C. H. June, Science 17 Apr 2020: DOI: 10.1126/science.abb8925
SARS-CoV-2 infection is believed to upregulate a variety of cytokines triggering the so called "cytokine storm", which correlates with COVID-19 disease progression. Patients infected with COVID-19 may die due to an excessive response of their immune system, characterized by the abnormal release of circulating cytokines and often vividly referred to as “cytokine storm”. Researchers are trying to understand this phenomenon and to identify a possible way to block it with targeted therapies. Interleukin-6 (IL-6) is a key pro-inflammatory cytokine and a plays a crutial role in the COVID-19-related immune response. Controlled clinical trials are under way worldwide to test IL-6 and IL-6R antagonists for the management of COVID-19 patients with severe respiratory complications.
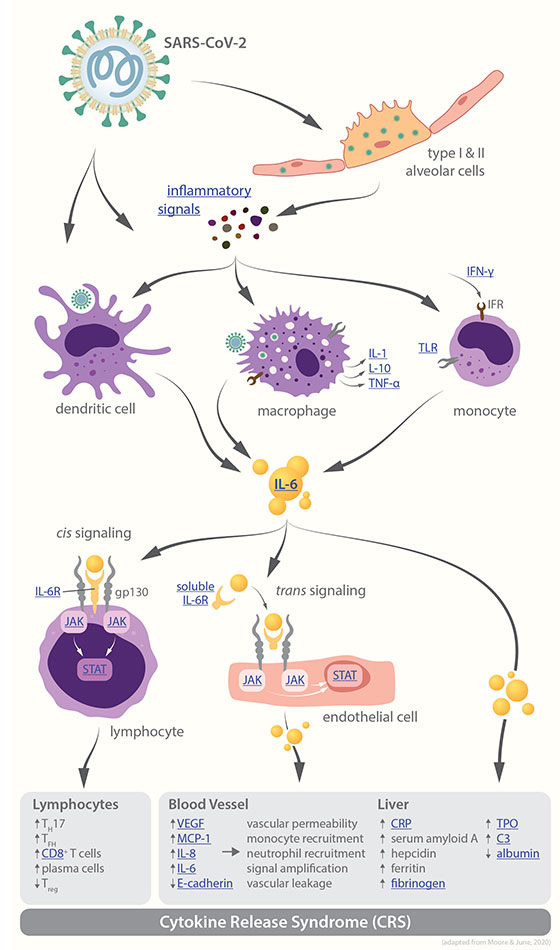
Graphic: V. Altounian / Science - Pathways leading to cytokine release syndrome
Coronavirus infection results in monocyte, macrophage, and dendritic cell activation. IL-6 release then instigates an amplification cascade that results in cis signaling with TH17 differentiation, among other lymphocytic changes, and trans signaling in many cell types, such as endothelial cells. The resulting increased systemic cytokine production contributes to the pathophysiology of severe COVID-19, including hypotension and acute respiratory distress syndrome (ARDS), which might be treated with IL-6 antagonists such as tocilizumab, sarilumab, and siltuximab.
 Designing a coronavirus vaccine - 10th March, 2020
Designing a coronavirus vaccine - 10th March, 2020
David Dowling, PhD, and Ofer Levy, MD, PhD (Michael Goderre/Boston Children’s Hospital, USA)
Focusing on adjuvants to boost immunity
The current antigen used for vaccine development (the part of the virus that the immune system “remembers”) is the coronavirus spike protein, named because it sits atop the spike of a coronavirus particle. Vaccine-induced antibodies, made by the immune system against the spike protein, can prevent infection.
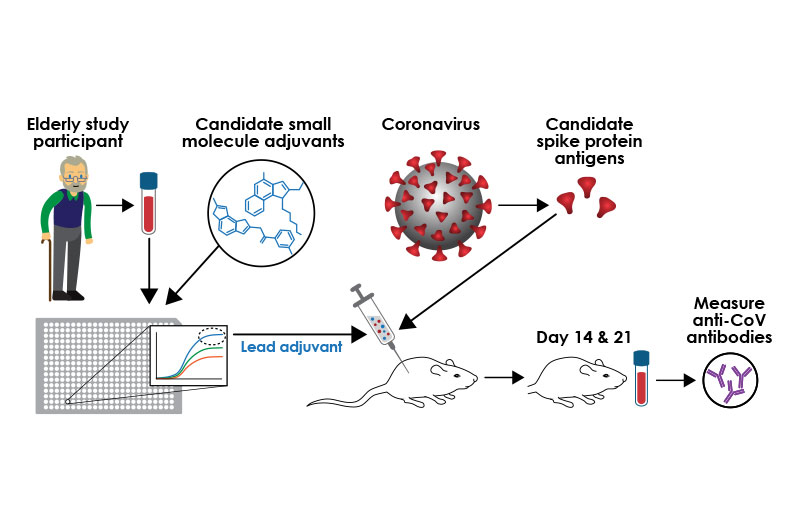
To develop a coronavirus vaccine targeted to an older population, researchers are using small molecule adjuvants together with the coronavirus spike antigen. Once the lead vaccine candidate is injected in mice, researchers will measure levels of antibodies against the virus. (Kristin Johnson/Boston Children’s Hospital)
Three COVID-19 vaccine concepts
It is estimate that more than 30 COVID-19 vaccine candidates are in development globally. These vaccines fall into three general types:
- RNA-based vaccines.
The first COVID-19 vaccines in development have used SARS-CoV-2 RNA. This approach is innovative and attractive, but even if proven effective it may be difficult to create hundreds of millions of doses, and each dose may be relatively expensive as it may require a fair amount of RNA. While there is less experience with human testing for this approach, it is possible that adjuvants may help enhance the immune response when such vaccines are administered to older populations and may reduce the amount of RNA needed in each vaccine.”
- DNA-based technology.
Like RNA-based vaccines, this promising approach uses the genetic material of the virus to produce a vaccine. You can make more vaccine doses with DNA than with RNA, but it is unclear whether the production could be rapidly scaled to meet the massive international demand. These nucleic acid vaccines have not yet been tested in elderly patients either.”
- Building on earlier coronavirus vaccines.
Learning from earlier vaccines from prior coronavirus outbreaks and making them more effective. If an adjuvant chosen specifically for optimal activity is added in an older population, not only does it work better in that group, but it may dramatically reduce the cost per vaccine dose by reducing the amount of antigen required.
Credit - Precision Vaccines Program, Boston Children’s Hospital, USA.
 Current novel coronavirus (2019-nCoV) outbreak
Current novel coronavirus (2019-nCoV) outbreak
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans. Based on experience with two other coronaviruses, SARS and MERS, person-to-person spread has continued to occur, and is currently spreading Globally. Countries affected and in lock-down include China and Italy and soon more could be on the list.
It may be possible to develop therapeutics to treat 2019-nCoV much more rapidly than vaccines hence many companies are investigating the efficacy of existing antivirals. The hunt to determine if mAbs against the SARS virus are effective against 2019-nCoV has started. Researchers are also developing mAbs based on the 2019-nCoV, starting with antibodies derived from blood drawn from patients diagnosed with this infectivity.
Therapeutics before vaccines
Some researchers are developing mRNA vaccine against 2019-nCoV by taking the gene for the spike protein S and inserting it into a mRNA platform to create a vaccine based on recombinant receptor-binding domain (RBD) of the SARS-CoV spike (S) protein. Because there are some similarities between the new virus and SARS, it is possible that SARS vaccine will cross-protect against the new virus because the sequence homology looks good so far. At Inovio's lab in San Diego, California, scientists are using a relatively new type of DNA technology to develop a potential vaccine with plans for it to enter human trials by early summer 2020 !
Availability of a vaccine on an emergency basis could take anything from 1-2 years.
Current tests for Coronavirus
Health professionals begin by swabbing a person’s nose or throat, collecting phlegm coughed up from the lungs, or squirting liquid into the nose, throat or lungs and collecting the liquid again for testing. Then, these samples are analyzed in a laboratory, where technicians must extract and purify the virus’s genetic material from the mucus, cell debris and other components of the samples. This usually is the biggest bottleneck in testing. Co-Diagnostics (CDC), a company based in Salt Lake City and Gujarat, India, that has developed its own coronavirus test.
All of the coronavirus tests being used by public health agencies and private labs around the world start with a technique called polymerase chain reaction, or PCR, which can detect tiny amounts of a virus’s genetic material. SARS-CoV-2, the virus that causes COVID-19, has RNA as its genetic material. That RNA must first be copied into DNA. After that, the PCR can begin. The process makes millions to billions of copies of selected segments of DNA. In the case of the coronavirus, the CDC’s original test scanned for three of the virus’s genes, but now tests for two. The World Health Organization’s test, developed by infectious disease researcher Christian Drosten at the Charité – Universitätsmedizin Berlin and colleagues, tests for three genes but is a bit different than the CDC tests. The PCR step typically takes 45 minutes to an hour. Some assays give instant yes / no answer, but others may also take time to analyze. Altogether, it may take about three hours to complete a single test, hence a high throughput system would be needed to test a large number of samples.
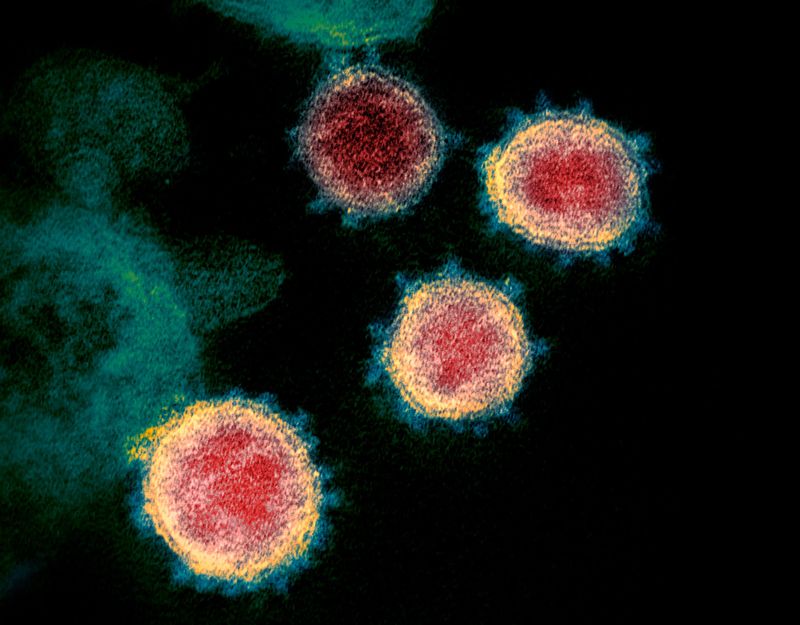
Credit: U.S. National Institutes of Health/AP/Shutterstock - Coronavirus taken with an electron microscope
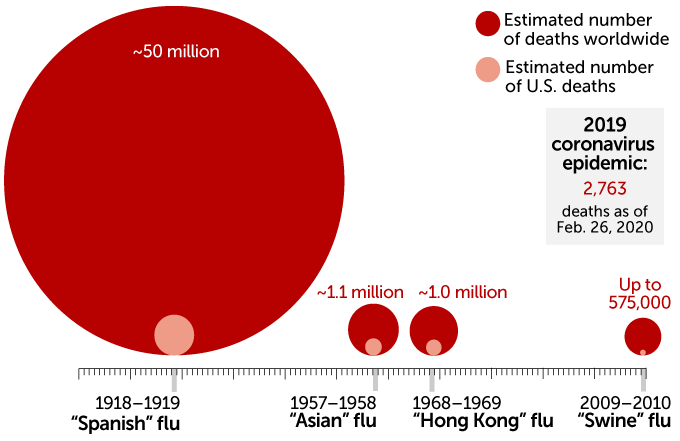
Estimated figures - CDC / Global Growing figures for 2019 - nCoV
 The COVID-19 coronavirus ourbreak and possible origins of the virus - take your pick !
The COVID-19 coronavirus ourbreak and possible origins of the virus - take your pick !
-
It was in November 9, 2015 that Wuhan laboratory in China announced that they had created a new virus from SARS-CoV. This goes to show that the Laboratory had been actively creating new viruses for all sort of use. Hence, it was not surprising that in December 2019 while the Chinese regime were downplaying the threat of the virus on the world stage, it was secretly scrambling to vanish all traces of the epidemic.
Subsequently, China's National Health Commission reportedly ordered all virus samples be destroyed and issued a 'no-publication order' about the virus. As part of a mass 'suppression and destruction of evidence', the state ordered samples of the virus to be destroyed in laboratories while the wet market was bleached to extinguish remnants of the disease. China had started censoring news of the virus on search engines from December 31 2019, deleting terms such as 'SARS variation, 'Wuhan Seafood market' and 'Wuhan Unknown Pneumonia.' It is public knowledge too that any manuscript on COVID-19 has now to go through a special Chinese review committee before it is allowed to be submitted to a foreign Journal – what do you make of all this?
- In other scenario, the virus evolved to its current pathogenic state through natural selection in a non-human host and then jumped to humans. This is how previous coronavirus outbreaks have emerged, with humans contracting the virus after direct exposure to civets (SARS) and camels (MERS). The researchers proposed bats as the most likely reservoir for SARS-CoV-2 as it is very similar to a bat coronavirus. There are no documented cases of direct bat-human transmission, however, suggesting that an intermediate host was likely involved between bats and humans.
- In another proposed scenario, a non-pathogenic version of the virus jumped from an animal host into humans and then evolved to its current pathogenic state within the human population. For instance, some coronaviruses from pangolins, armadillo-like mammals found in Asia and Africa, have an RBD structure very similar to that of SARS-CoV-2. A coronavirus from a pangolin could possibly have been transmitted to a human, either directly or through an intermediary host such as civets or ferrets.
Recent research found that the RBD portion of the SARS-CoV-2 spike proteins had evolved to effectively target a molecular feature on the outside of human cells called ACE2, a receptor involved in regulating blood pressure. The SARS-CoV-2 spike protein was very effective at binding the human cells.
 The Oxford Vaccine Centre’s COVID-19 vaccine trial
The Oxford Vaccine Centre’s COVID-19 vaccine trial
The Oxford Vaccine Centre’s COVID-19 vaccine trial is being run by the Jenner Institute and Oxford Vaccine Group. The team, who started work on developing a vaccine to prevent COVID-19. A chimpanzee adenovirus vaccine vector (ChAdOx1), developed at Oxford’s Jenner Institute, was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine as it can generate a strong immune response from one dose and it is not a replicating virus, so it cannot cause an ongoing infection in the vaccinated individual. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects, from 1 week to 90 years of age, in vaccines targeting over 10 different diseases.
Coronaviruses have club-shaped spikes on their outer coats. Immune responses from other coronavirus studies suggest that these are a good target for a vaccine. The Oxford vaccine contains the genetic sequence of this surface spike protein inside the ChAdOx1 construct. After vaccination, the surface spike protein of the coronavirus is produced, which primes the immune system to attack the coronavirus if it later infects the body. At the same time as preparing for and conducting the first clinical trial, production of the vaccine is being scaled up ready for larger trials, and potentially, future deployment - fingers crossed !
 GSK2618960, an anti-IL7-R monoclonal antibody, in healthy volunteers -
GSK2618960, an anti-IL7-R monoclonal antibody, in healthy volunteers -
GlaxoSmithKline (GSK, UK) is developing the monoclonal antibody GSK2618960 as a potential medicine for auto-immune diseases. In those diseases, the body’s own immune system attacks healthy organs and tissues by mistake. Some of the cells in the immune system (lymphocytes) attack when a protein, called IL7, binds to a receptor for IL7 (IL7R) on the surface of those cells. The antibody GSK2618960 blocks IL7R, which might reduce the damage caused by lymphocytes in auto-immune diseases.
 Surveillance Testing - why it is a must and what is the difference between diagnostic testing (PCR) and surveillance testing?
Surveillance Testing - why it is a must and what is the difference between diagnostic testing (PCR) and surveillance testing?
Diagnostic testing and surveillance are critical, complementary strategies to combat pathogen outbreaks. Diagnostic testing helps clinicians manage patients and surveillance is required to manage populations.
- Diagnostic testing provides important yes/no answers for individual patients so that appropriate management can be provided.
- Surveillance helps public health officials track the path of the epidemic, understand transmission routes, determine the rate of viral evolution, and understand if the virus is changing in ways that impact therapeutic effectiveness.
- Sequencing identified SARS-Cov-2 as the virus causing the cluster of respiratory illnesses in Wuhan. Deep shotgun sequencing is unbiased and can be performed on nasal swabs and other samples to look for non-host genomic material, which can then be assembled into potential causative agents. The sequence rapidly identified the virus as one closely resembling a coronavirus endemic in bats, but also revealed a mutation in the protein involved in attaching to host cells that helped explain the ability of the virus to infect human cells.
- Sequencing can determine how quickly the virus is adapting as it spreads. It appears that SARS-CoV-2 achieves two mutations per month as it spreads. This information allows public health officials to identify how likely the virus is to avoid detection from established PCR assays and become resistant to therapies.
 Favipiravir (also known as Avigan) a Japanese flu drug appears ‘effective’ in COVID-19 treatment in Chinese clinical trials
Favipiravir (also known as Avigan) a Japanese flu drug appears ‘effective’ in COVID-19 treatment in Chinese clinical trials
Based on results of clinical trials conducted with affected patients in both Wuhan and Shenzhen by Chinese medical authorities, Japanese-made flu drug favipiravir (also known as Avigan) has been shown to be effective in both reducing the duration of the COVID-19 virus in patients and to have improved the lung conditions of those who received treatment with the drug.
The trials involved 340 patients in total, and since the drug has already been developed and approved for use in treating flu, it has a “high degree of safety,” according to China science and technology ministry official Zhang Xinmin. The tests showed a reduction in the period during which patients tested positive for the new coronavirus from 11 days down to just four, and showed improvements in the lung condition of around 91 percent of patients treated with favipiravir, compared to just 62 percent for those without among the trial participants.
Still, a treatment that is effective in reducing the duration of the presence of the virus even in milder cases, and in lessening the impacts in moderate symptomatic patients, would be a huge benefit to the ongoing fight against the coronavirus. Any approvals for use of favipiravir would, of course, require further clinical testing, followed by approval of widespread use by each country’s relevant medical treatment regulating body.
Other drug treatments have been tested for COVID-19 treatment, and are in the process of development, but no antiviral has yet been approved or created specifically for dealing with the new coronavirus. Other drugs that have shown early promising signs include remadesivir, a compound developed by Gilead Science.



