- HOME
- Vienna 2024
- Lithuania 2023
- 16th CEEPC Prague 2022
- Proteome & Proteomics
- Proteomics Potentiality
- Precision Medicine & Cancer
- Proteomics and COVID-19
- Big Data & AI
- Spotlight Lithuania
- Humanity
- Meeting Reports & Tributes
- Country Profiles
- Proteomic Snippets
- Enabling Advances
- Spotlight Czech Republic
- Spotlight Poland
- 13th CEEPC - Ustron, Poland
- Spotlight Romania
- 12th CEEPC - Bucharest
- Spotlight Slovakia
- Spotlight Macedonia
- Sports Medicine
- Contacts & Copyrights
Precision Medicine and Cell & Gene Therapy

Precision Medicine & Personalized Medicine

'One size does not fit all'
The Digital Revolution in Precision medicine - Challenges and Opportunities.
- New medical technologies have saved and improved millions of lives in recent decades. Yet the digital technologies that are transforming contemporary health care, such as digital medical devices, applications of medical software, and artificial intelligence (AI) tools to diagnose and treat patients, would have been unrecognizable few years ago.
- Many digital health applications (apps) meet the formal definition of a medical device, yet their software-based nature means that evaluating their safety and efficacy are incompatible. This mismatch underlies many of today's challenges in commercializing new products and adoption of healthcare technology in a safe and meaningful way.
Precision Medicine and CEEPC challenges < CLICK TO READ
Credit: “Cancer “Liquid Biopsy” Blood Test Gets Expanded FDA Approval was originally published by the National Cancer Institute.”
FDA has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects genetic changes in tumor DNA in the blood and can help match patients to potential treatments. In instances where the blood test does not identify the specific genetic change that means a patient can receive a corresponding targeted therapy FDA advised that the patient’s tumor tissue should be analyzed “to determine if the specific mutations and alterations are present.”
Synopsis
What do we currently have in medicine?
Electronic medical records (EMRs) - a digital version of the paper charts in the clinician’s office. An EMR contains the medical and treatment history of the patients with advantages over paper records including:
- Tracking of data over time
- Easy identification of which patients are due for preventive screenings or checkups
- Checking of patient parameters such as blood pressure readings, vaccinations, drug treatment monitoring, biological monitoring, etc..
- Monitoring and improving overall quality of care within the health service
Experience with a doctor or GP today is almost identical to what it was 20 years ago - except that instead of looking at you, the doctor is begrudgingly documenting your exchanges into EHRs. Taking your blood pressure, a prescription and smile are the only ‘take-aways’ to make you feel better - was it worth a visit !
Biological testing is just the start of the process !
Corona virus testing has unmasked our limitations !
 Every day around the world, millions of people take the wrong medications and suffer adverse reaction or death and this is unacceptable. We currently do nothing about it as we watch helplessly. Our genomic make-up and information about medications prescribed should have precise information about the outcome – but currently it is a ‘lottery’ or a gamble. Today, both sets of these data are readily available and at our disposal. Moving forward, ‘Machine learning’ and ‘AI’ will eventually close the loop to harness the predictive power of the data to ensure that one does not receive the wrong medication. We are now talking about the futuristic pharmacy.
Every day around the world, millions of people take the wrong medications and suffer adverse reaction or death and this is unacceptable. We currently do nothing about it as we watch helplessly. Our genomic make-up and information about medications prescribed should have precise information about the outcome – but currently it is a ‘lottery’ or a gamble. Today, both sets of these data are readily available and at our disposal. Moving forward, ‘Machine learning’ and ‘AI’ will eventually close the loop to harness the predictive power of the data to ensure that one does not receive the wrong medication. We are now talking about the futuristic pharmacy.
Similarly, future Emergency departments and Surgeries will be very different. Rather than waiting for symptomatic escalation, many medical events will be detected and intercepted upstream. Emergency services powered by AI, wearables, and digital streaming data will facilitate this. As we grapple with healthcare’s monumental challenges, we should consider whether our biggest opportunities are not just ones of medical science or technology, but ones closer to the ground—rethinking how to use technology and data to redefine healthcare itself.
'End-to-end informatics solutions' for clinical applications remain the greatest unmet need and much more needs to be done if we are to promise a better health-care service ! Through machine learning and artificial intelligence, we have the ability to reason about and utilize data at an unprecedented scale in order to predict, prevent, and treat disease more effectively.
Demands to treat the individual rather than the average human and the advances in artificial intelligence (AI) is ushering in precision medicine or personalized medicine where tailoring predictive, preventive and treatment strategies to the individual is a priority. Since protein expression is dynamic and changes in relation to disease onset, severity or response to therapy are difficult to understand, proteomics stands to play a pivotal role in characterizing a disease at protein level. Additionally, the trial and error approach to prescribing medication can be dangerous and costly – not only from a financial perspective but from a health perspective. Proteo-genomics provides the ability to take genetic insights coupled with lifestyle and medical information to help select the medication that will work the best for each individual.
Proteomic technologies have progressed over the last decade allowing in principle the comprehensive analysis of expressed proteins in time and space. Until now, quantitative proteomics has been pin-pointing minor differences in the protein levels between normal and pathological samples. There is now an urgent need for sophisticated ‘enabling technologies’ to identify structural differences in proteins introduced by mutations or structural variations induced by post-translational modifications or protein truncation that are associated with a disease. Additionally, comprehensive characterization of the small molecule metabolites in biological systems and biological applications of the Metabolome together with the Proteome in precision medicine of the patient, stands to revolutionize global health.
The complexity of the data generated has also been a stumbling block in understanding diseases because proteome analysis does not provide a simple ‘yes/no’ answer but rather requires deep interpretation. To this end, utilization of data and information from various ‘multi-omics’ studies including metabolomics, together with AI in the hands of skilled researchers, can have significant societal impact.
 CEEPC's careful balance between excellence and focus on societal needs holds the key to its success. It is evident from CEEPC’s progress that it is ready for the next decade of excitement and expectations of multifaceted proteomics in Central and Eastern Europe. Additionally, in the era of emerging personalized medicine where treatment selection for each patient is becoming individualized, CEEPC and proteomics is expected to play a significant role moving forward for the benefit of mankind.
CEEPC's careful balance between excellence and focus on societal needs holds the key to its success. It is evident from CEEPC’s progress that it is ready for the next decade of excitement and expectations of multifaceted proteomics in Central and Eastern Europe. Additionally, in the era of emerging personalized medicine where treatment selection for each patient is becoming individualized, CEEPC and proteomics is expected to play a significant role moving forward for the benefit of mankind.
Scientists recently mapped the human epigenome for the first time. The epigenome is made up of chemicals and proteins that can attach to DNA and modify its function by turning our genes on and off. An individual's lifestyle and environment factors like whether they smoke or what their diet looks like, can prompt sometimes deadly changes in their epigenome that can cause cancer. Mapping the epigenome may help scientists understand how tumors develop and cancer spreads.
Current medical and research challenges include:
Cancer, Neurological, Metabolic, Cardio-vascular, etc.....
CAR T-cell
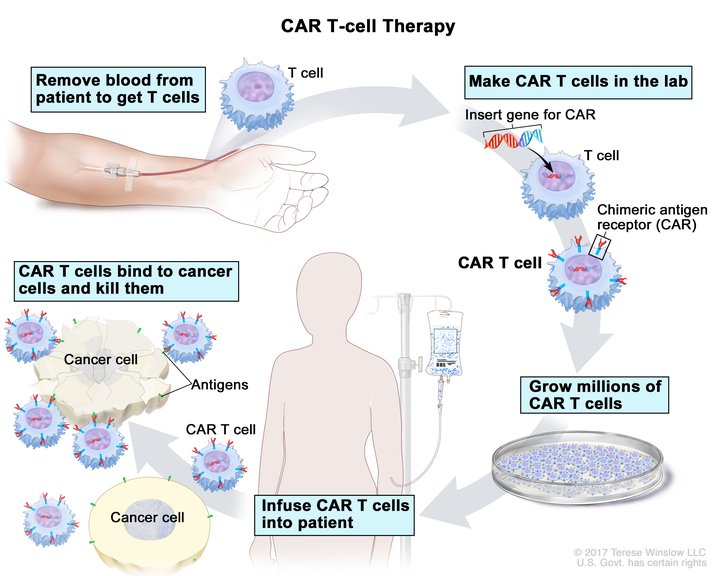
Efforts are on-going with genetic engineering to help the body’s immune system target cancer cells using CAR (chimeric antigen receptor) T-cell therapy. CAR-T cells are T-cells, immune cells of the body that are reprogrammed to identify specific surface signatures that define specific types of cancer cells. It allows these immune agents to seek and destroy cancer cells with great accuracy and with fewer side effects than the traditional chemotherapy or radiation.
* Tisagenlecleucel, marketed as Kymriah, is a treatment for B-cell acute lymphoblastic leukemia (ALL) and Diffuse large B-cell lymphoma (DLBCL), which uses the body's own T cells to fight cancer (adoptive cell transfer).
* Kymriah is a type of advanced therapy medicine called a ‘gene therapy product’. This is a type of medicine which works by delivering genes into the body. Kymriah contains the active substance tisagenlecleucel (consisting of genetically modified white blood cells).
Currently, it is being tried out for treating two types of blood cancer:
* B-cell acute lymphoblastic leukaemia (ALL), in children and young adults up to 25 years of age whose cancer did not respond to previous treatment, has come back two or more times, or has come back after a transplant of stem cells;
* Diffuse large B-cell lymphoma (DLBCL) in adults whose cancer has come back or did not respond after two or more previous treatments.
While in their infancy, these techniques show great promise for future therapies and are currently being tried out as shown above.
Gene Therapy
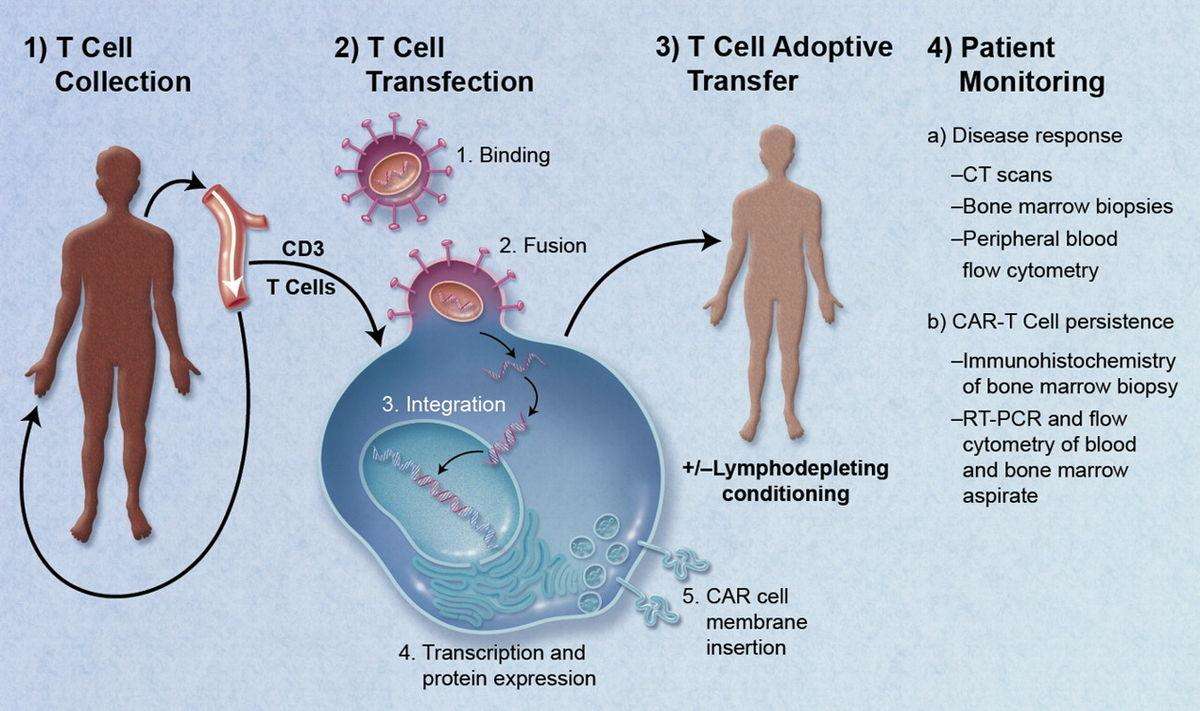
Gene therapy is an experimental technique that uses genes to treat or prevent disease. In the future, this technique may allow treatment of a disorder by inserting a gene into a patient’s cells instead of using drugs or surgery. Several approaches are being tried out in gene therapy, including:
a) Replacing a mutated gene that causes disease with a healthy copy of the gene.
b) Inactivating, or “knocking out,” a mutated gene that is functioning improperly.
c) Introducing a new gene into the body to help fight a disease.
Although gene therapy is a promising option for a number of diseases (including inherited disorders, some types of cancer, and certain viral infections), the technique remains risky and is still under study to ensure that it will be safe and effective. Gene therapy is currently being tested only for diseases that have no other cures.
Reality and Reasons
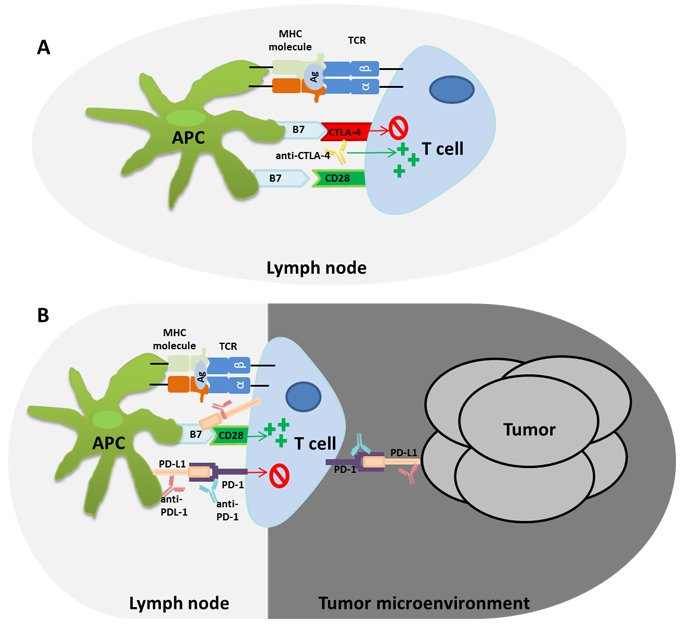
'On-going' studies show the complexity of cancer and the need to understand the microenvironment and the inter-relationships of various triggers and signals !
Cancer cell-cell / cell - matrix interactions that remain elusive !
Protein as Drug treatment
In cancer cells, one protein that stands out on their surface is the B- cell maturation antigen and it is possible that the future of treating the most advanced blood cancer multiple myeloma may lie with this single protein on tumor cells. This protein is now an appealing target to wipe out the cancer without affecting healthy tissue and three different initiatives include:
1) Activating the body's own immune cells to identify and kill diseased cells;
2) Using another protein that binds with the antigen to deliver a killer dose of chemotherapy;
3) Using a dual-acting protein that will pull cancer-fighting immune cells into cancerous cells.
Protein as a drug, chemical molecules as drugs or combinations of with the above mentioned therapies may lead to a possible solution in the near future. The chances of a success remain much lower than that of a failure !
Radiotherapy and novel Radiopharmaceuticals
Researchers are now designing and testing radiopharmaceuticals for a range of cancers as diverse as melanoma, lung cancer, colorectal cancer, and leukemia. Any tumor that has a targetable molecule on the surface of its cells and a good blood supply, sufficient to deliver drugs, could potentially be treated with radiopharmaceuticals. The idea of linking a cancer-targeting molecule with a molecule that kills cancer cells is not new either. For example, several drugs called antibody-drug conjugates, in which an antibody that binds to specific cancer cells is linked to a toxic drug, have been in use.
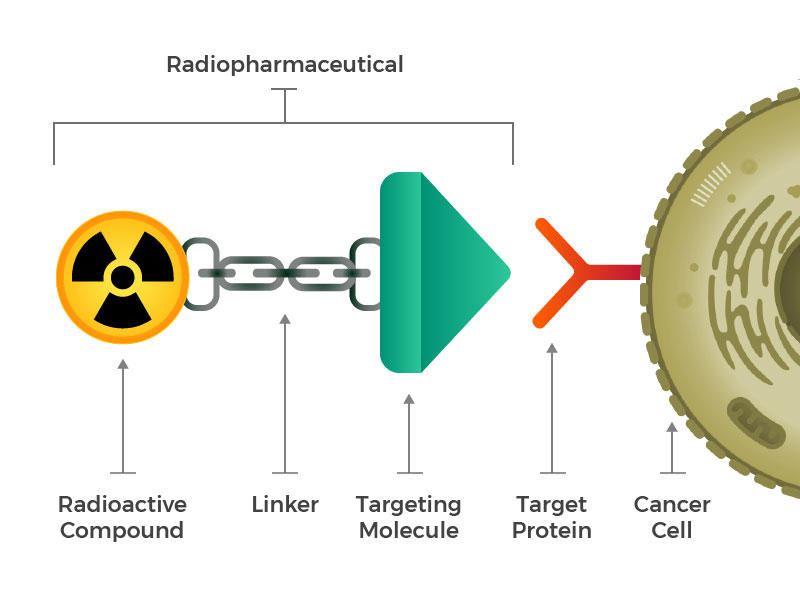
Credit: National Cancer Institute
Radiopharmaceuticals consist of a radioactive molecule, a targeting molecule, and a linker that joins the two.
The game-changer is the FDA approved Lutetium Lu 177-dotatate (Lutathera) for treatment of certain cancers.
Many tumors are “cold” tumors in that immune cells do not recognize them, or don’t work properly in the microenvironment around tumors. But when radiation kills cancer cells, proteins and DNA from those cells can spill into the bloodstream for immune cells to see, which may allow the immune cells to recognize and kill other cancer cells throughout the body. Radiation therapy may also make the tumor microenvironment more hospitable to immune cells. Together, these effects can turn a cold tumor into a “hot” tumor: one that has an abundance of immune cells and may be responsive to immunotherapy drugs.
Metabolomics
Metabolomics is the study of the metabolome, including identification of biochemical and molecular characteristics of the metabolome, interactions among different metabolites or between metabolites and genetic/environmental factors and evaluation of biochemical mechanisms related different pathophysiological processes. Metabolome contains all metabolites derived from sugars, lipids, proteins, and nucleic acids in a given biological system, tissue, cell, or body-fluid. The metabolites in a metabolome interact in enzymatic reaction systems to form metabolic network systems. The alteration in metabolome is associated with multiple factors, including genetic, environmental or dietary factors. The progress in Metabolomics is highly dependent on high-sensitivity reproducible technologies and approaches necessary to maximize the coverage of variations in the metabolome leading to discovery of effective biomarkers and / or proteins still to be identified. Such progress will significantly add to ‘Personalized Medicine’. Newer technologies and Mass Spectrometry have a special role to play in this area of research moving forward.
"mRNA" vaccines for Cancer, dementia, flu and heart diseases
"mRNA" is a truly transformational technology and we have seen its life-saving power during the pandemic where ‘rapid cutting-edge vaccines’ were vital in the response to the COVID pandemic.
Developing the next generation of mRNA vaccines will be crucial in boosting our ability to prevent and respond to a wide range of respiratory diseases in the future. Whilst conventional vaccines are produced using weakened forms of the virus, mRNAs use only the virus's genetic code. No actual virus is needed to create an mRNA vaccine. This means the rate at which the vaccine can be produced is accelerated. The up and coming new vaccines using the messenger RNA (mRNA) technology, will teach the body's cells how to make a protein that will trigger an immune response. It is hoped the technology will go on to be used to tackle a wide range of other diseases including cancer, dementia, flu and heart disease. This next phase of vaccine development has already started with a milestone date of 2025!

.................what is the reality and why are we failing in finding an ideal solution to cancers !
CEEPC is focused on improving prevention, early detection, diagnosis, and treatment of cancer by enhancing the understanding of the molecular mechanisms of cancer. Advancing proteome science, "mRNA" vaccine science for Cancer, dementia, flu and heart diseases and the understanding of proteogenomic inter-relationship together with metabolomics, may help to further the cause for all above scenarios mentioned.
