- HOME
- Vienna 2024
- Lithuania 2023
- 16th CEEPC Prague 2022
- Proteome & Proteomics
- Proteomics Potentiality
- Precision Medicine & Cancer
- Proteomics and COVID-19
- Big Data & AI
- Spotlight Lithuania
- Humanity
- Meeting Reports & Tributes
- Country Profiles
- Proteomic Snippets
- Enabling Advances
- Spotlight Czech Republic
- Spotlight Poland
- 13th CEEPC - Ustron, Poland
- Spotlight Romania
- 12th CEEPC - Bucharest
- Spotlight Slovakia
- Spotlight Macedonia
- Sports Medicine
- Contacts & Copyrights
Bioinformatics, AI and Big Data - our shortcoming !

The idea that cutting-edge technologies like Nvidia and ChatGPT, are the most superior and that you would have to pay for access seems to be fading very fast with the emergence of DeepSeek. On January 20, 2025, DeepSeek-R1 was released which is ‘open access’, totally changing the perspective of superiority and limited access. Firms like OpenAI, Meta, Google, Apple, and Microsoft have to face up to this new competitor DeepSeek – a start-up that develops open-source AI models, meaning the developer community can inspect and improve the software and anyone can download it for free !

Artificial intelligence (AI)
Artificial intelligence based computation and clinical interpretation and reporting capabilities stands to chance the way we approach disease prognosis and treatment for an individual. AI - based computation and interpretation solution can only happen if the big data information technology and its integration with healthcare processes (including ‘electronic health record’ (EMR / EHR), X-ray, imaging data, MRI scans, NMR, etc…..) together with research data from Proteomic, Genomics and Metabolomics is combined and crunched to develop and provide artificial intelligence (AI)-based computation and interpretation solutions
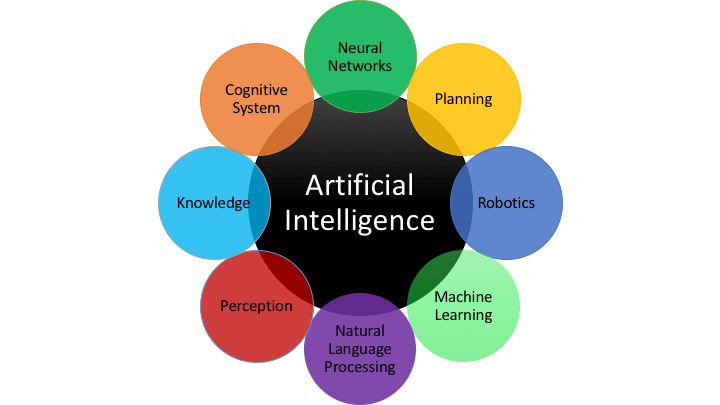
What is AI ?
Big Data
- Data collection is now easier than ever – but with such high volumes of data at our fingertips, it is becoming difficult to ensure we’re using it well
- Before accessing available data, it’s important to understand what was collected and how, to ensure that it’s fit for purpose
- New kinds of data require new governance, new standards, and new interfaces in order to ensure good quality and comprehensibility
- Big data will be increasingly important for a wide range of applications – but only if our technologies, and our methods, keep up!
Challenges ahead in Big Data Analysis & Bioinformatics
- Currently, we are lacking ‘end to end’ Bioinformatic solutions focused on clinical research and applications and this remains the unmet need moving forward. Despite joint efforts from different industries to develop end to end products and solutions, the progress has been slow. Many have now turned to strategic alliances, partnerships, and collaborations to enhance capabilities to offer an integrated and interoperable solutions to the scientific and medical community.
The following would certainly help including:
- High adoption of cloud-based solutions to support ever-increasing high-end data storage client requirements, with public and private cloud solutions integrated into service offerings;
- Developing informatics products for precision medicine and clinical and molecular diagnostics
- Combination of multi-omics data for interpretation of disease scenarios;
- Driving big data analytics for clinical interpretation;
- Provision of high-speed computation and data analysis tools
- High-throughput sequencing and population-scale genome sequencing;
- A move away from core informatics solutions toward 'sequencing-to-report' offerings with informatics platforms integrated with novel technologies
- A 'software-as-a-service' (SAAS), with Proteomics, Genomic & Metabolomic 'sample-to-clinical' report capability.

Machine learning
Machine learning models for 'Cancer - Drug' studies
Machine learning techniques hold immense promise for better drug response predictions, but most have not reached clinical practice due to their lack of interpretability and their focus on monotherapies. Now, interpretable deep learning model of human cancer cells trained on the responses of large number of tumor cell lines to library of drugs are being developed. Tumor genotypes induce states in cellular subsystems that are integrated with drug structure to predict response to therapy and, simultaneously, learn biological mechanisms underlying the drug response. Predictions can be accurate in cell lines and also stratify clinical outcomes. Analysis of mechanisms may lead directly to the design of synergistic drug combinations, which can be validated systematically by combinatorial CRISPR, drug-drug screening in vitro, and patient-derived xenografts. Such models provide a blueprint for constructing interpretable models for predictive medicine. Below is one example:
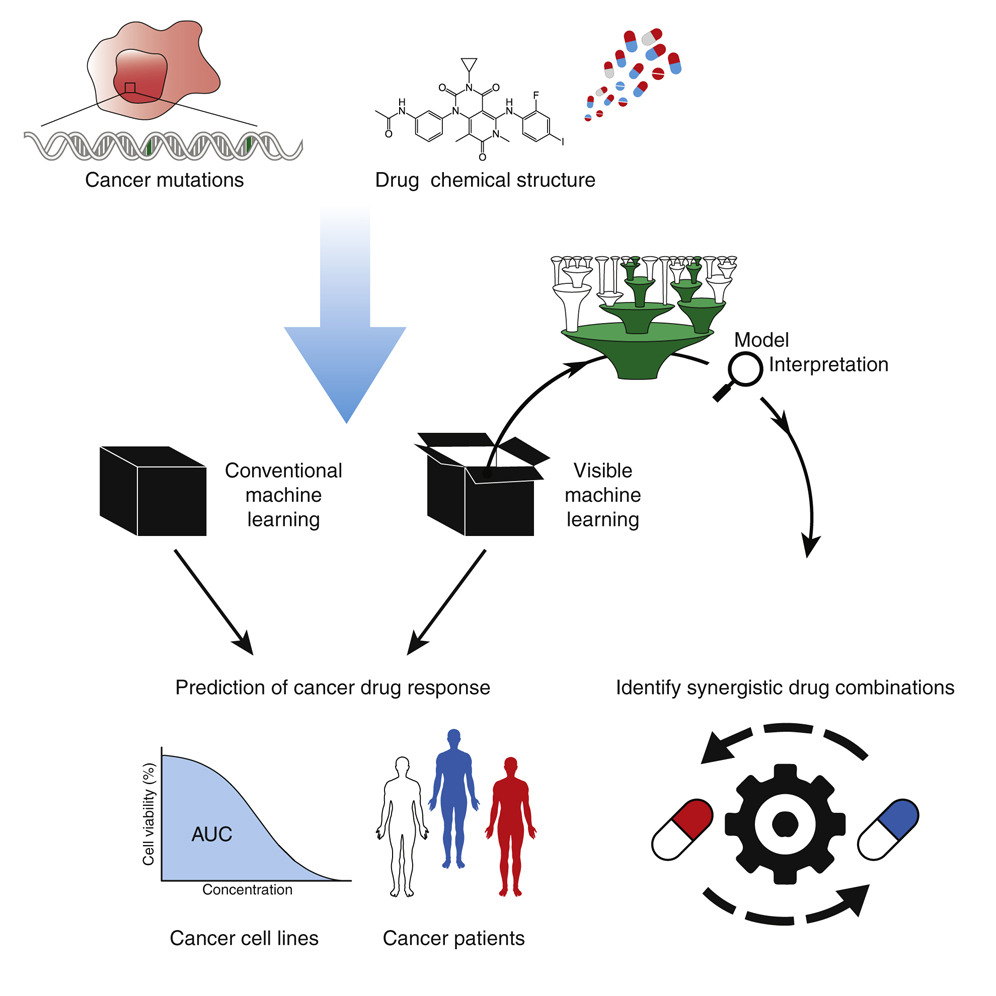
Credit: Brent M. Kuenzi, et al, Cancer Cell, 2020; DOI: 10.1016/j.ccell.2020.09.014
 ...............reality is that researchers predict that in the next few years there will be examples of targets discovered through machine learning and ‘in silico' methods that unravel biology that wasn't appreciated before. Drug discovery is time consuming, hampered by human biology, incomplete knowledge of disease states as well as high costs. Hence, AI and the use of machine learning or other artificial intelligence approaches to comb through genomic databases, or draw links between molecular structures and drug activity, is an alternative way forward.
...............reality is that researchers predict that in the next few years there will be examples of targets discovered through machine learning and ‘in silico' methods that unravel biology that wasn't appreciated before. Drug discovery is time consuming, hampered by human biology, incomplete knowledge of disease states as well as high costs. Hence, AI and the use of machine learning or other artificial intelligence approaches to comb through genomic databases, or draw links between molecular structures and drug activity, is an alternative way forward.
In practice, AI has yet to make much of an impact on drug discovery success rates or speed and discovering new targets is only the start – as we know, many biological targets have been considered "undruggable" due to challenges in medicinal chemistry! Whilst many talk about AI in drug discovery, there are only a hand-full of Pharama companies actually starting to think about it. It will take a new type of pharma company to embark on AI from scratch and with a totally different & innovative approach if we are to see any positive outcome.
Interestingly, COVID-19 is highlighting our inability to properly track, trace or test!
Our digital technology needs to be significantly improved...........
 Putting computing technologies together with a naked human brain, you get something that's smarter...and the result is that we, supplemented by our technology, are actually capable of accomplishing much more complex tasks than we could with our un-supplemented biological abilities.
Putting computing technologies together with a naked human brain, you get something that's smarter...and the result is that we, supplemented by our technology, are actually capable of accomplishing much more complex tasks than we could with our un-supplemented biological abilities.
